基于超高分辨率和超长深度光学相干层析成像技术的悬尾鼠航天相关神经眼综合征研究
doi: 10.11728/cjss2023.05.2023-05-yg11 cstr: 32142.14.cjss2023.05.2023-05-yg11
Study on Spaceflight-associated Neuro-ocular Syndrome with the Rat Tail Suspension by ULOCT and UHROCT
-
摘要: 为研究航天相关神经眼综合征(SANS)相关特征参数与微重力的相关性,以Sprague-Dawley(SD)大鼠为悬尾动物模型,构建了超长扫描深度的光学相干断层扫描(ULOCT)装置,测量大鼠眼部特征参数(角膜、前房、晶状体厚度和玻璃体腔深度及眼轴长度),以及视网膜超高分辨率(UHROCT)测量装置,测量大鼠眼底亚层结构参数(视神经层、视网膜内层、视网膜外层、脉络膜和巩膜的厚度)。结果表明大鼠悬尾1月后,眼底彩照未见明显视盘水肿等症状,但OCT结果显示大鼠眼结构发生明显改变:角膜变薄(p<0.01),眼轴缩短(p<0.05),视网膜外层变薄(p<0.01)。这些特征参数变化(尤其是眼轴明显缩短)展现了SANS亚临床前期的症状。本文为后续微观层面的SANS分子机制研究提供了宏观动物模型以及在体眼部测量新方法。
-
关键词:
- 微重力 /
- 航天相关神经眼综合征 /
- 光学相干断层成像 /
- 眼轴缩短
Abstract: To investigate the correlation between the relevant characteristic parameters of Spaceflight-Associated Neuro-ocular Syndrome (SANS) and microgravity, Sprague-Dawley (SD) rats with a tail-suspended animal model were utilized. Ultra-Long Scan Depth Optical Coherence Tomography (ULOCT) system was set up to measure the characteristic parameters of the rat eyes (corneal thickness, anterior chamber depth, lens thickness, vitreous chamber depth, and axial length), and an Ultra-High-Resolution Optical Coherence Tomography (UHROCT) system to measure the sublayer structural parameters of the rat fundus (retinal nerve layer, inner retinal layer, outer retinal layer, choroid, and sclera thickness). The results came from the fundus photographs showed that there were no obvious symptoms of optic disc edema. However, the results came from ULOCT and UHROCT showed the significant changes in the rat eye structure: corneal thinning (p<0.01), axial shortening (p<0.05), and outer retinal thinning (p<0.01). These changes of characteristic parameters, especially the significant axial shortening, exhibited subclinical symptoms of SANS. This study provides a macroscopic animal model and a novel in vivo ocular measurement method for investigating the molecular mechanisms of SANS at the microscopic level in future researches. -
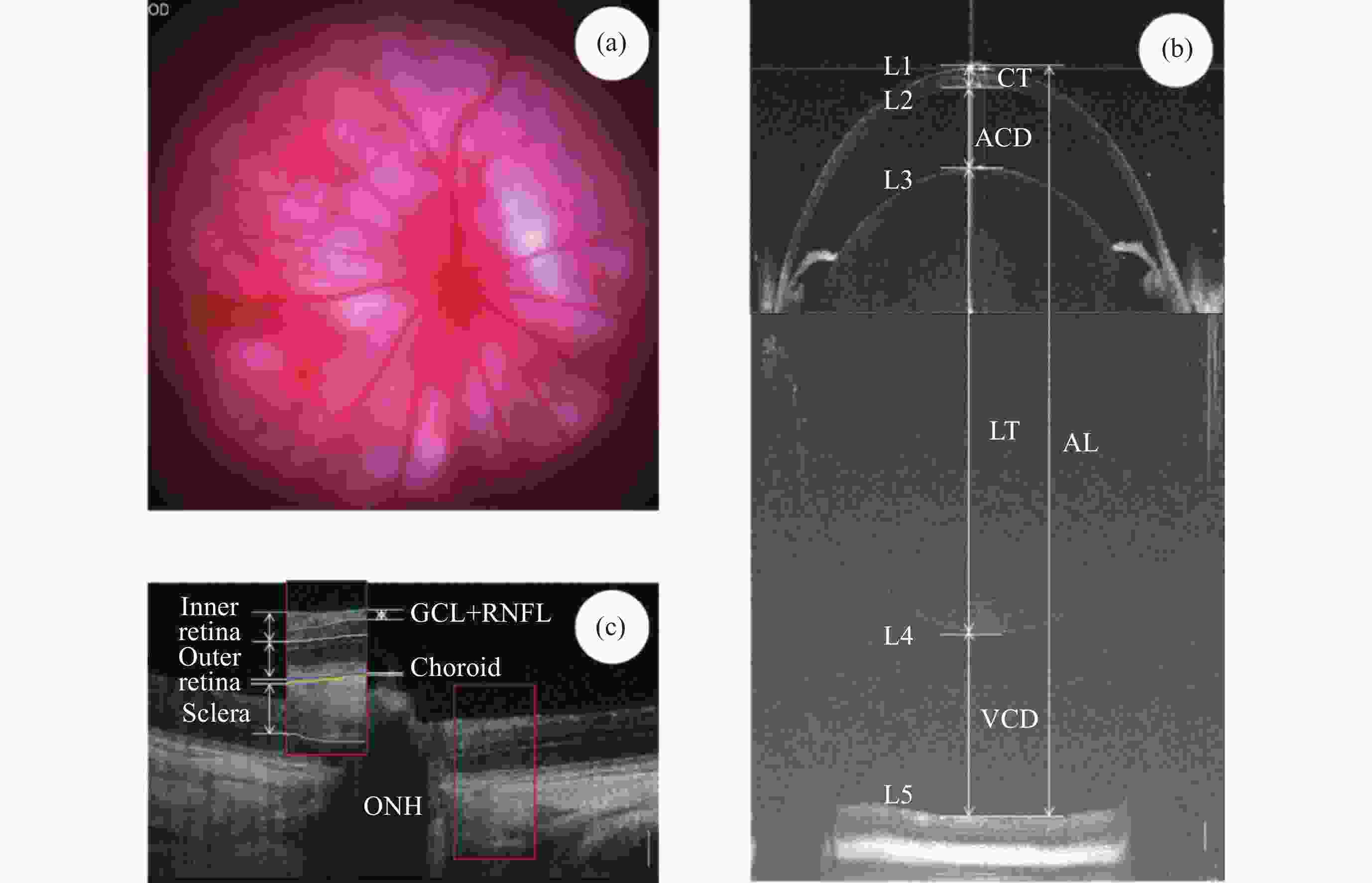
图 2 大鼠眼成像及分析。(a)大鼠眼底彩照,OD表示右眼,(b)大鼠超长深度OCT全眼成像, (c)大鼠超高分辨率OCT眼底成像及分层界限和层次定义。白色竖线为200 μm标尺
Figure 2. Schematic representation of rat ocular imaging and analysis. (a) Rat fundus color photograph (OD represents the right eye), (b) rat ultra-long depth OCT imaging of the entire eye, (c) rat high-resolution OCT fundus imaging with layer boundaries and definitions, with the white vertical line representing a 200 μm scale
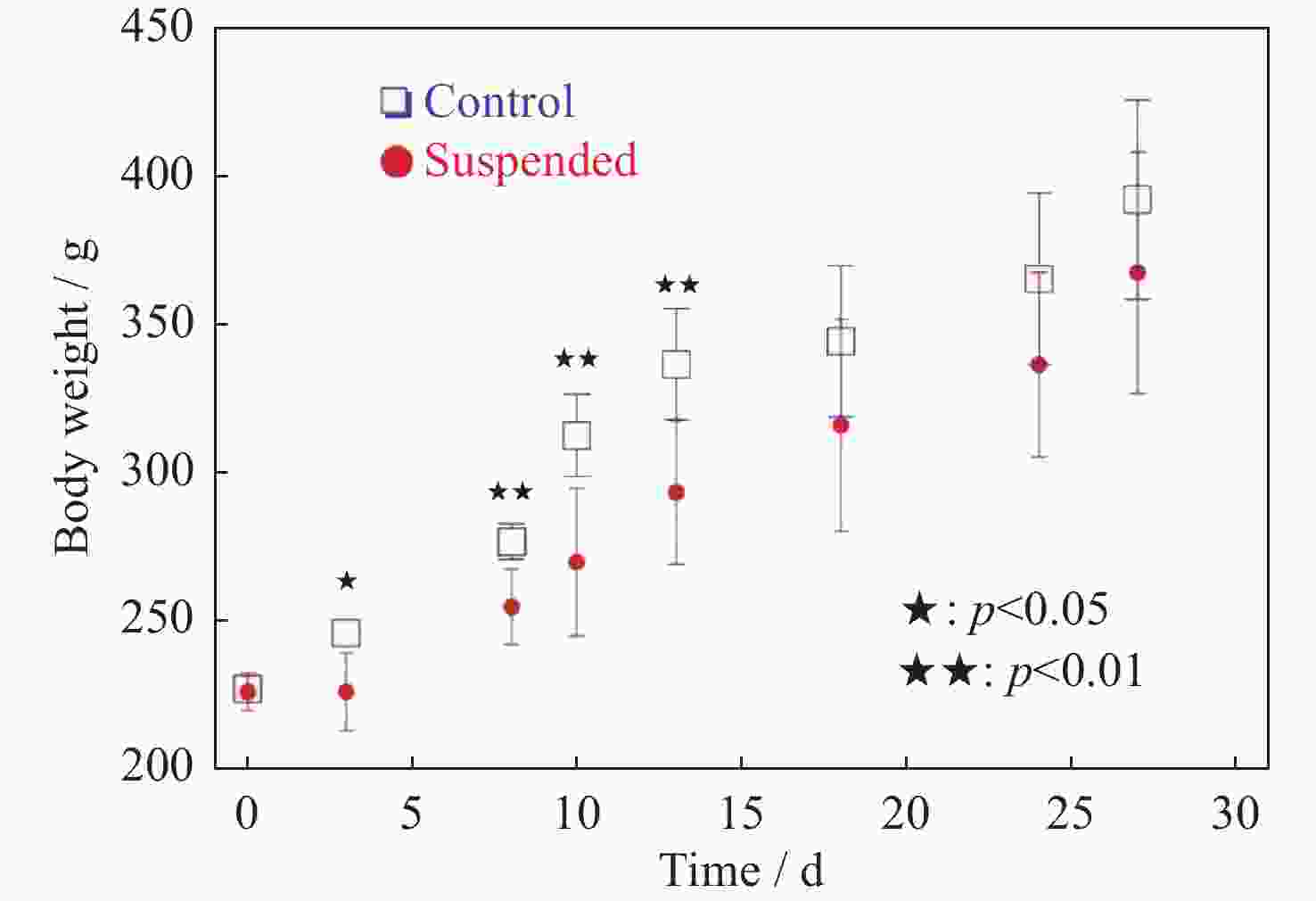
图 3 大鼠体重随悬尾天数的变化统计(统计数量n=6。数据显示第18天后二者的体重无统计差异)
Figure 3. Statistical rat body weight over the duration of tail suspension(The data indicated that there were no statistically significant differences in body weight between the two groups after the 18th day, the number of statistical samples for each group n is equal to 6)
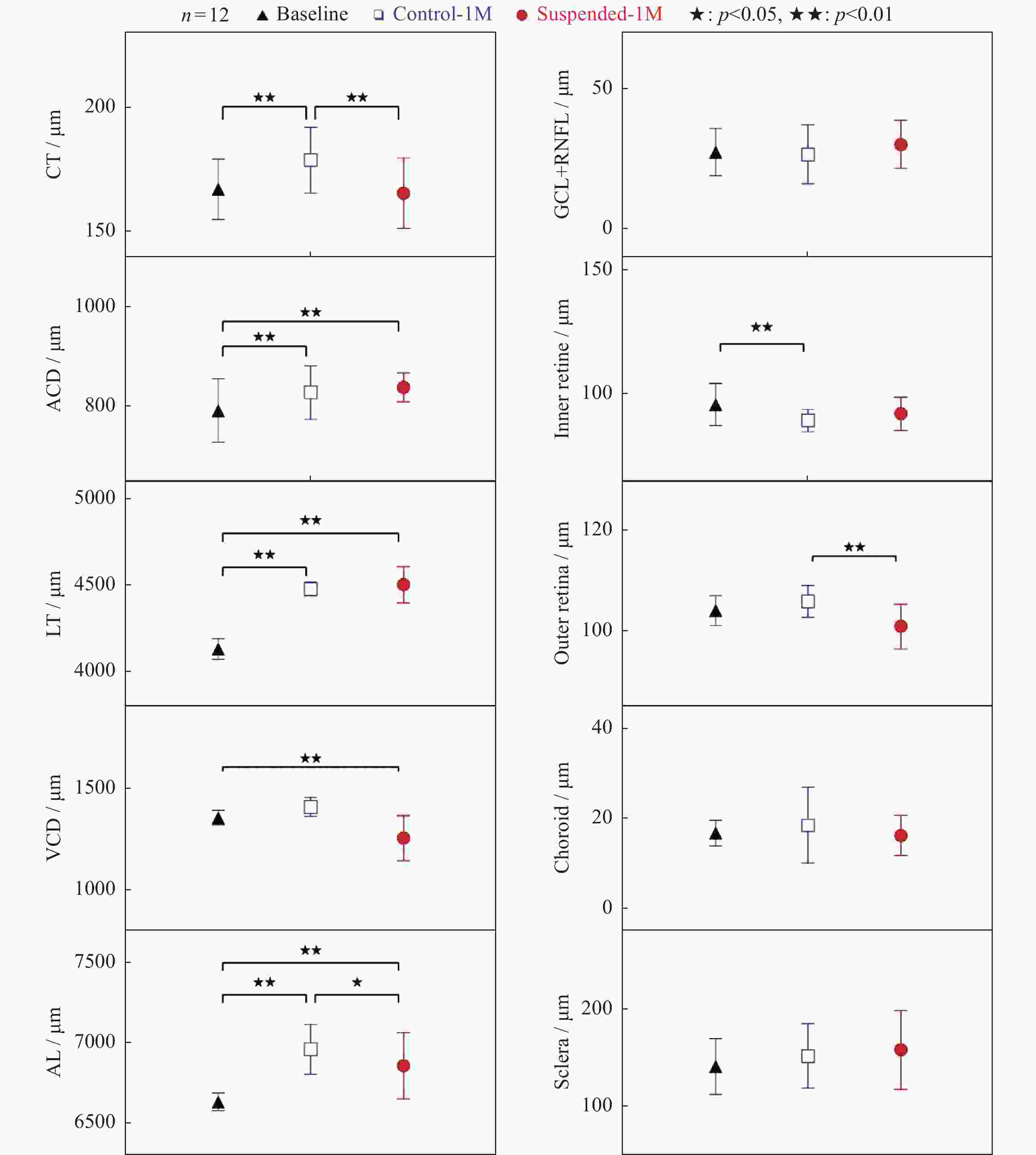
图 4 大鼠眼部特征参数(a)及眼底亚层结构参数统计分析(b)。数据表明悬尾一个月后, 眼轴明显缩短且视网膜外层厚度显著下降
Figure 4. Statistical graph of rat ocular feature parameters (a) and sublayer parameters of the fundus (b). The data indicated that after suspension of one month there was a significant decrease in axial length and outer retina thickness
-
[1] MACIAS B R, PATEL N B, GIBSON C R, et al. Association of long-duration spaceflight with anterior and posterior ocular structure changes in astronauts and their recovery[J]. JAMA Ophthalmology, 2020, 138(5): 553-559 doi: 10.1001/jamaophthalmol.2020.0673 [2] MADER T H, GIBSON C R, PASS A F, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight[J]. Ophthalmology, 2011, 118(10): 2058-2069 doi: 10.1016/j.ophtha.2011.06.021 [3] LEE A G, TARVER W J, MADER T H, et al. Neuro-ophthalmology of space flight[J]. Journal of Neuro-Ophthalmology, 2016, 36(1): 85-91 doi: 10.1097/WNO.0000000000000334 [4] LEE A G, MADER T H, GIBSON C R, et al. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: a review and an update[J]. npj Microgravity, 2020, 6: 7 doi: 10.1038/s41526-020-0097-9 [5] MADER T H, GIBSON C R, MILLER N R, et al. An overview of spaceflight-associated neuro-ocular syndrome (SANS)[J]. Neurol. India, 2019, 67(2): S206-S211 [6] KHOSSRAVI E A, HARGENS A R. Visual disturbances during prolonged space missions[J]. Current Opinion in Ophthalmology, 2021, 32(1): 69-73 doi: 10.1097/ICU.0000000000000724 [7] RIASCOS R F, KAMALI A, HAKIMELAHI R, et al. Longitudinal analysis of quantitative brain MRI in astronauts following microgravity exposure[J]. Journal of Neuroimaging, 2019, 29(3): 323-330 doi: 10.1111/jon.12609 [8] ZHANG L F, HARGENS A R. Spaceflight-induced intracranial hypertension and visual impairment: pathophysiology and countermeasures[J]. Physiological Reviews, 2018, 98(1): 59-87 [9] SUBRAMANIAN P S. Spaceflight-associated neuro-ocular syndrome and increased intracranial pressure-are we closer to understanding the relationship?[J]. JAMA Ophthalmology, 2023, 141(2): 176-177 doi: 10.1001/jamaophthalmol.2022.5686 [10] ROBERTS D R, COLLINS H R, AL KASAB S. The importance of the intracranial compartment in the development of spaceflight-associated neuro-ocular syndrome[J]. JAMA Ophthalmology, 2022, 140(1): 98-99 [11] ONG J, TARVER W, BRUNSTETTER T, et al. Spaceflight associated neuro-ocular syndrome: proposed pathogenesis, terrestrial analogues, and emerging countermeasures[J]. British Journal of Ophthalmology, 2023, 107(7): 895-900 [12] 李向前, 任泽, 严伟明, 等. 长期微重力暴露所致眼损伤疾病动物模型的构建[J]. 实验动物科学, 2018, 35(3): 52-58 doi: 10.3969/j.issn.1006-6179.2018.03.010LI Xiangqian, REN Ze, YAN Weiming, et al. Construction of an animal model of ocular damage caused by long-term microgravity exposure[J]. Laboratory Animal Science, 2018, 35(3): 52-58 doi: 10.3969/j.issn.1006-6179.2018.03.010 [13] ZHAO H W, ZHAO J, HU L N, et al. Effect of long-term weightlessness on retina and optic nerve in tail-suspension rats[J]. International Journal of Ophthalmology, 2016, 9(6): 825-830 [14] DAI X F, YE S M, CHEN X P, et al. Rodent retinal microcirculation and visual electrophysiology following simulated microgravity[J]. Experimental Eye Research, 2020, 194: 108023 doi: 10.1016/j.exer.2020.108023 [15] 宫玉波, 许永杰, 赵宏伟, 等. 14 d尾吊模拟失重大鼠眼底血流动力学及眼轴变化研究[J]. 航天医学与医学工程, 2019, 32(5): 401-405GONG Yubo, XU Yongjie, ZHAO Hongwei, et al. Study of simulated weightlessness by tail suspension on changes of ocular hemodynamics and axis in rats[J]. Space Medicine & Medical Engineering, 2019, 32(5): 401-405 [16] 宫玉波, 赵宏伟, 宋飞龙, 等. 微重力环境下大鼠眼底血流动力学及视网膜、脉络膜厚度的变化[J]. 解放军医学杂志, 2021, 46(1): 7-10 doi: 10.11855/j.issn.0577-7402.2021.01.02GONG Yubo, ZHAO Hongwei, SONG Feilong, et al. Changes of ocular fundus hemodynamics and retinal and choroidal thickness after tail suspension for 2 weeks in rats[J]. Medical Journal of Chinese People’s Liberation Army, 2021, 46(1): 7-10 doi: 10.11855/j.issn.0577-7402.2021.01.02 [17] 戴旭锋, 保金华, 陈晓萍, 等. 模拟失重对成年小鼠闪光视网膜电图和视网膜微循环的影响[J]. 国际眼科杂志, 2020, 20(1): 27-31DAI Xufeng, BAO Jinhua, CHEN Xiaoping, et al. Impact of simulated microgravity on flash electroretinogram and retinal microcirculation in adult mice[J]. International Eye Science, 2020, 20(1): 27-31 [18] STENGER M B, LAURIE S S, SADDA S R, et al. Focus on the optic nerve head in spaceflight-associated neuro-ocular syndrome[J]. Ophthalmology, 2019, 126(12): 1604-1606 doi: 10.1016/j.ophtha.2019.09.009 [19] MINAKARAN N, DE CARVALHO E R, PETZOLD A, et al. Optical coherence tomography (OCT) in neuro-ophthalmology[J]. Eye, 2021, 35(1): 17-32 doi: 10.1038/s41433-020-01288-x [20] 黄芙蓉, 颜婷婷, 熊薇薇, 等. 超长扫描深度的频域光学相干断层扫描在小鼠眼球生物学测量中的应用[J]. 中华眼视光学与视觉科学杂志, 2013, 15(6): 347-353 doi: 10.3760/cma.j.issn.1674-845X.2013.06.008HUANG Furong, YAN Tingting, XIONG Weiwei, et al. Biometric measurement of the mouse eyes using extended scan depth spectral domain optical coherence tomography[J]. Chinese Journal of Optometry Ophthalmology and Visual Science, 2013, 15(6): 347-353 doi: 10.3760/cma.j.issn.1674-845X.2013.06.008 [21] WANG Y H, JIANG H, SHEN M X, et al. Quantitative analysis of the intraretinal layers and optic nerve head using ultra-high resolution optical coherence tomography[J]. Journal of Biomedical Optics, 2012, 17(6): 066013 doi: 10.1117/1.JBO.17.6.066013 [22] 陈杰, 马进, 丁兆平, 等. 一种模拟长期失重影响的大鼠尾部悬吊模型[J]. 空间科学学报, 1993, 13(2): 159-162CHEN Jie, MA Jin, DING Zhaoping, et al. A modified tail-suspension model for simulating long-term weightlessness[J]. Chinese Journal of Space Science, 1993, 13(2): 159-162 [23] PARK H N, QAZI Y, TAN C, et al. Assessment of axial length measurements in mouse eyes[J]. Optometry and Vision Science, 2012, 89(3): 296-303 doi: 10.1097/OPX.0b013e31824529e5 [24] MOREY-HOLTON E R, GLOBUS R K. Hindlimb unloading rodent model: technical aspects[J]. Journal of Applied Physiology, 2002, 92(4): 1367-1377 doi: 10.1152/japplphysiol.00969.2001 [25] MAO X W, BYRUM S, NISHIYAMA N C, et al. Impact of spaceflight and artificial gravity on the mouse retina: biochemical and proteomic analysis[J]. International Journal of Molecular Sciences, 2018, 19(9): 2546 doi: 10.3390/ijms19092546 [26] MAO X W, NISHIYAMA N C, BYRUM S D, et al. Characterization of mouse ocular response to a 35-day spaceflight mission: evidence of blood-retinal barrier disruption and ocular adaptations[J]. Scientific Reports, 2019, 9(1): 8215 doi: 10.1038/s41598-019-44696-0 [27] MAO X W, NISHIYAMA N C, BYRUM S D, et al. Spaceflight induces oxidative damage to blood-brain barrier integrity in a mouse model[J]. The FASEB Journal, 2020, 34(11): 15516-15530 doi: 10.1096/fj.202001754R [28] MAO X W, PECAUT M J, STODIECK L S, et al. Spaceflight environment induces mitochondrial oxidative damage in ocular tissue[J]. Radiation Research, 2013, 180(4): 340-350 doi: 10.1667/RR3309.1 [29] MAO X W, SANDBERG L B, GRIDLEY D S, et al. Proteomic analysis of mouse brain subjected to spaceflight[J]. International Journal of Molecular Sciences, 2019, 20(1): 7 [30] ROQUE-TORRES G D, NISHIYAMA N C, STANBOULY S, et al. Assessment of global ocular structure following spaceflight using a micro-computed tomography (micro-CT) imaging method[J]. J. Vis. Exp., 2020, DOI: 10.3791/61227 [31] SARUNIC M V, YAZDANPANAH A, GIBSON E, et al. Longitudinal study of retinal degeneration in a rat using spectral domain optical coherence tomography[J]. Optics Express, 2010, 18(22): 23435-23441 doi: 10.1364/OE.18.023435 [32] SCHMUCKER C, SCHAEFFEL F. A paraxial schematic eye model for the growing C57 BL/6 mouse[J]. Vision Research, 2004, 44(16): 1857-1867 doi: 10.1016/j.visres.2004.03.011 [33] 菲利普·纳尔逊. 从光子到神经元: 光、成像和视觉[M]. 舒咬根, 黎明译. 北京: 科学出版社, 2021NELSON P. From Photon to Neuron: Light, Imaging, Vision[M]. SHU Yaogen, LI Ming trans. Beijing: Science Press, 2021 [34] LIU N, WU J L, CHEN Y X, et al. Channels that cooperate with TRPV4 in the brain[J]. Journal of Molecular Neuroscience, 2020, 70(11): 1812-1820 doi: 10.1007/s12031-020-01574-z [35] GRACE M S, BONVINI S J, BELVISI M G, et al. Modulation of the TRPV4 ion channel as a therapeutic target for disease[J]. Pharmacology & Therapeutics, 2017, 177: 9-22 [36] GARCIA-ELIAS A, MRKONJIĆ S, JUNG C, et al. The TRPV4 channel[M]//NILIUS B, FLOCKERZI V. Mammalian Transient Receptor Potential (TRP) Cation Channels. Berlin, Heidelberg: Springer, 2014: 293-319 [37] ZHU A Q, LIN Y, HU X B, et al. Treadmill exercise decreases cerebral edema in rats with local cerebral infarction by modulating AQP4 polar expression through the caveolin-1/TRPV4 signaling pathway[J]. Brain Research Bulletin, 2022, 188: 155-168 doi: 10.1016/j.brainresbull.2022.08.003 -
-






 下载:
下载: