模拟微重力效应破坏线粒体–纺锤体–染色体的协同互作诱发小鼠卵母细胞减数分裂异常
doi: 10.11728/cjss2025.05.2025-yg03 cstr: 32142.14.cjss.2025-yg03
Simulated Microgravity Effects-induced Disruption of Mitochondria-spindle-chromosome Coordination Causes Meiosis Defects in Mouse Oocytes
-
摘要: 采用随机定位仪模拟微重力效应, 研究重力方向连续随机改变对小鼠卵母细胞减数分裂的影响. 利用芯片装载GV期卵母细胞, 通过随机定位仪进行小鼠卵母细胞成熟培养, 分别对GV, GVBD, Pro-MI, MI, MII各时期的线粒体、纺锤体和染色体进行荧光成像, 结果显示, 与正常重力条件相比, 模拟微重力效应导致卵母细胞成熟率显著下降32.75% (p<0.01). 荧光成像显示, 模拟微重力效应加剧了MI期卵母细胞核周线粒体环状聚集现象, 同时使得MII期细胞质中线粒体无规律聚集占比显著增多至71.88%. 此外, 模拟微重力效应引发MI期纺锤体多极化, 导致染色体排列紊乱, 最终导致MII期纺锤体组装和染色体中板宽度异常, 分别为57.58% (p<0.05) 和15.63 μm (p<0.0001). 本研究表明, 模拟微重力效应通过干扰线粒体–纺锤体–染色体的动态协调, 导致减数分裂异常和卵母细胞质量下降, 本研究对进一步研究重力条件变化对卵母细胞减数分裂的影响机制具有重要意义.Abstract: Meiosis is essential for oocyte maturation and embryonic development, representing a critical factor in mammalian reproduction. Consequently, assessing the impact of space microgravity on this process is paramount for evaluating reproductive health during long-term space missions. This article used a Random Positioning Machine (RPM) to simulate microgravity effects, examining how random changes in orientation relative to the gravity vector affect mouse oocyte meiosis. This study aims to provide critical biological references for further investigation into the damage mechanisms of space microgravity environments on oocyte meiotic division. Germinal Vesicle (GV)-stage mouse oocytes were encapsulated in Polydimethylsiloxane (PDMS) chip chambers to ensure stable culture conditions and precise positional control during RPM operation. Oocytes were cultured under RPM (RPM group) and static Normal Gravity (NG group) conditions. Meiotic progression was tracked and quantitatively analyzed at five key developmental stages: GV (0 h), GV Breakdown (GVBD, 2 h), Pro-Metaphase I (Pro-MI, 5 h), Metaphase I (MI, 8 h), and Metaphase II (MII, 16 h). Mitochondrial distribution, spindle morphology, and chromosome alignment were quantified through confocal laser microscopy coupled with fluorescent probes. The results showed that RPM condition reduced oocyte maturation rates by 32.75% compared to Normal Gravity (NG) controls (p<0.01). Mitochondrial dynamics exhibited stage-specific perturbations: perinuclear clustering at MI-stage (70.00% vs. 41.18% in NG) and disorganized aggregation patterns in 71.88% of MII-stage oocytes. In addition, spindle assembly and chromosome alignment were also disrupted: multipolar spindles during MI-stage caused disordered chromosome arrangement. At MII, RPM group oocytes displayed exacerbated spindle defects (57.58% abnormality rate vs. 22.32% in NG, p<0.05) and widened equatorial plates (15.63 μm vs. 7.55 μm, p<0.0001). These findings suggest that the simulated microgravity effect leads to abnormal meiosis and the decline of oocyte quality through tripartite disruption of mitochondrial-spindle-chromosomal coordination. This study significantly contributes to understanding how gravity changes affect oocyte meiosis.
-
Key words:
- Oocyte /
- Microgravity /
- Mitochondria /
- Spindle /
- Chromosome
-
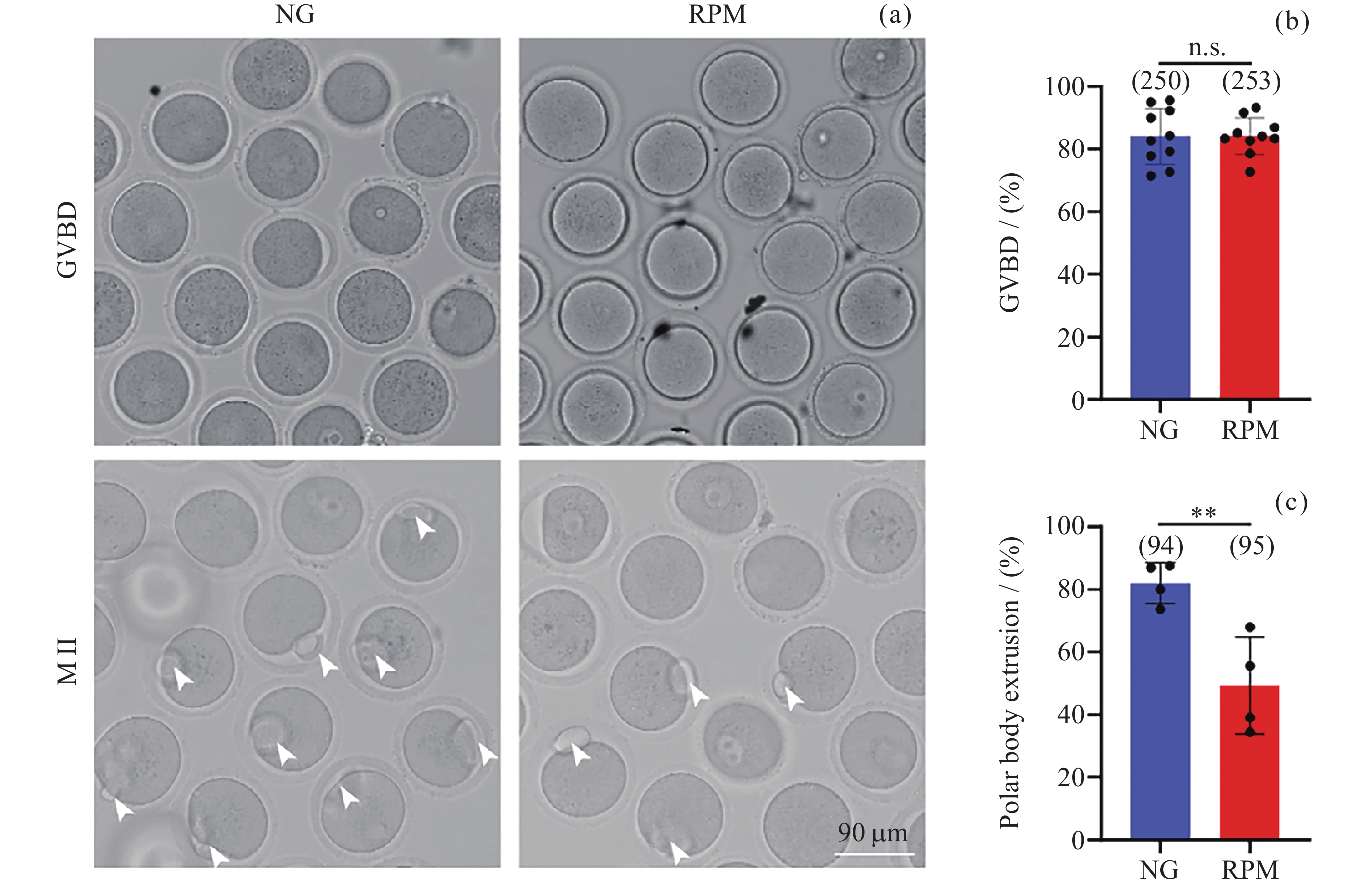
图 1 NG组和RPM组卵母细胞GVBD、Metaphase II时期明场图像, 白色箭头表示排出极体的成熟卵母细胞 (a). NG组和RPM组卵母细胞GVBD率 (b) 与第一极体排出率 (c) 的定量分析
Figure 1. Representative bright field images in GVBD and Metaphase II oocytes under NG and RPM conditions, where the white arrows indicate mature oocytes with extruded polar bodies (a). Quantitative analysis of oocyte GVBD rates (b) and first polar body extrusion rates (c) in NG group and RPM group, respectively
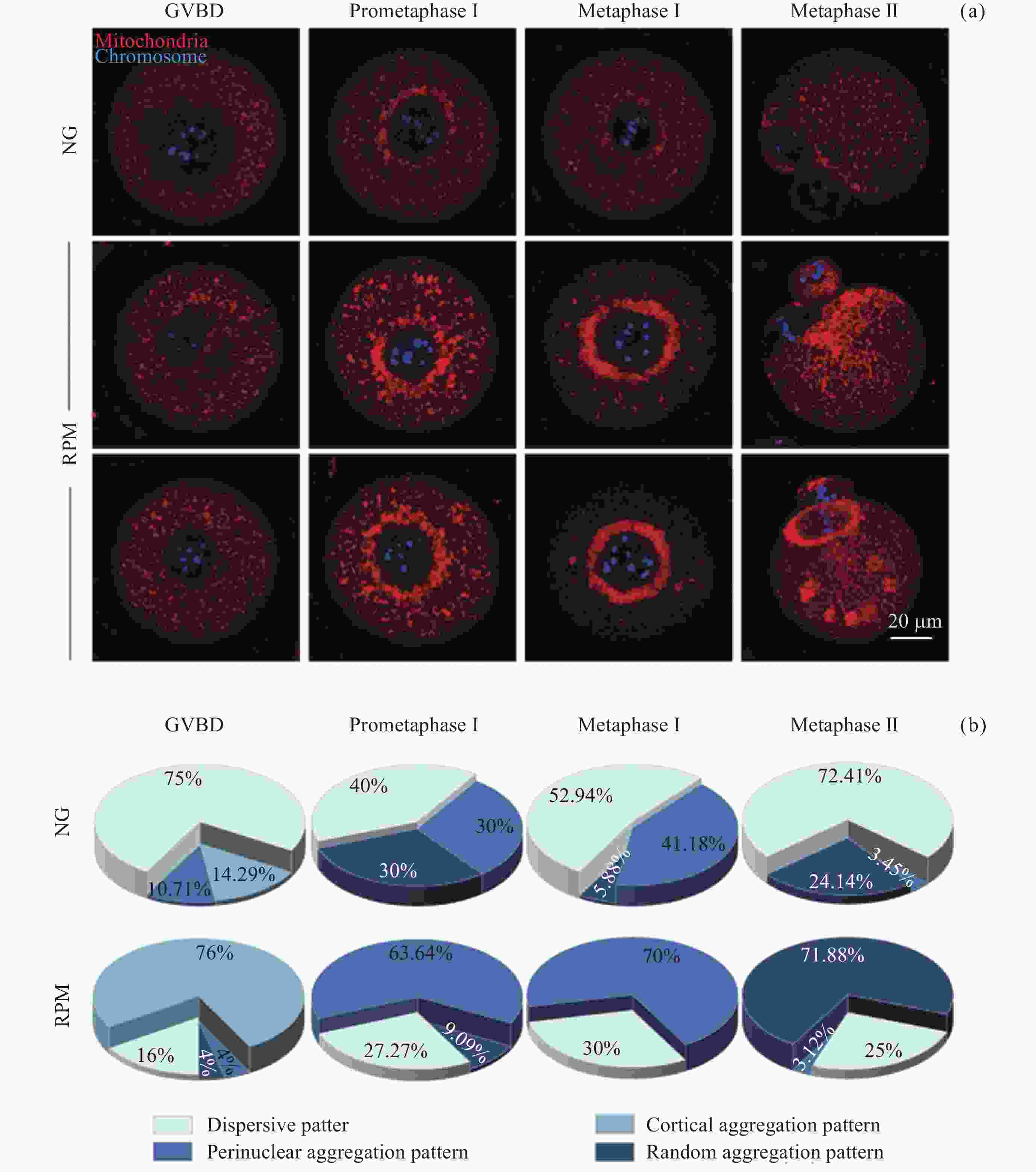
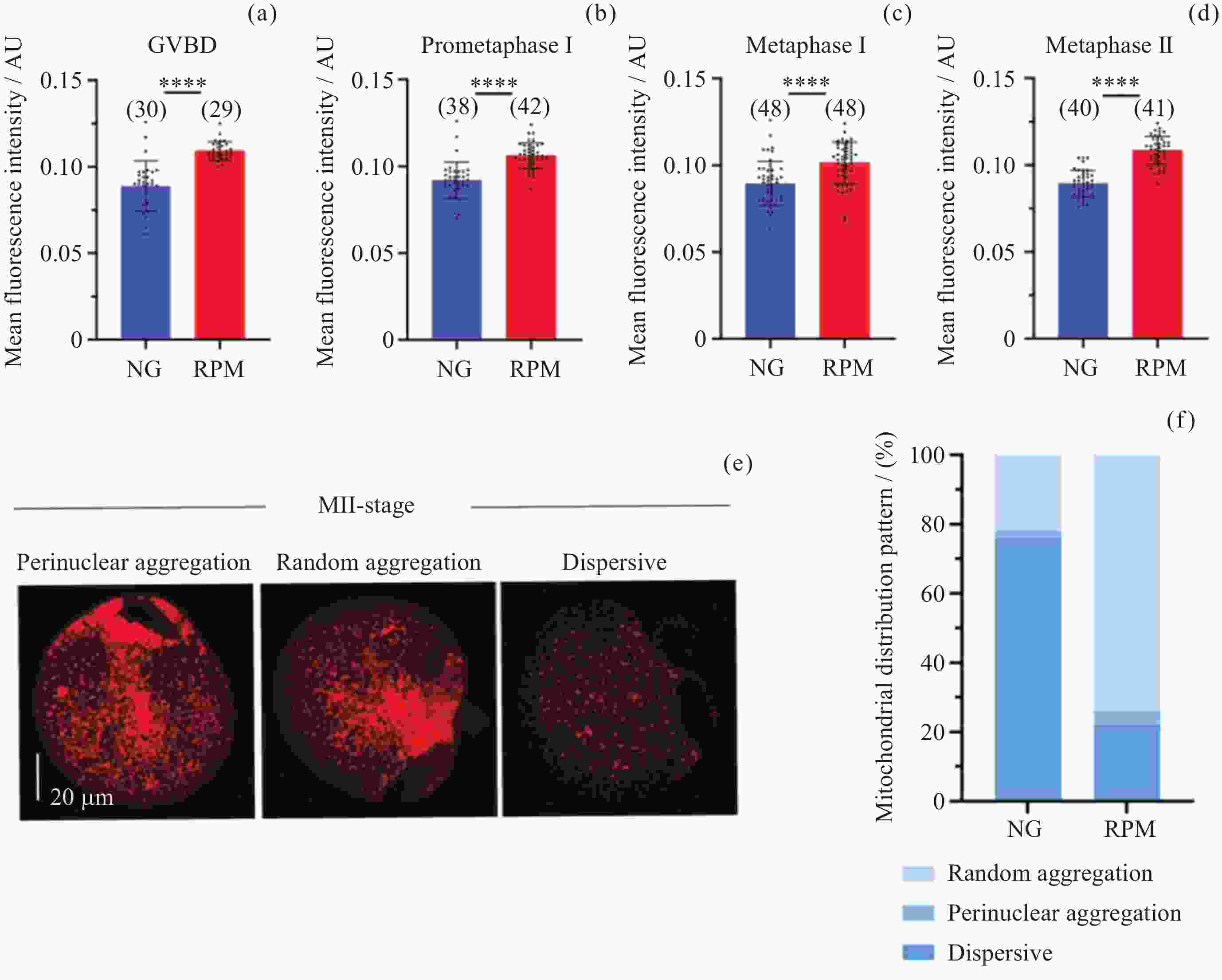
图 3 NG组和RPM组卵母细胞在GVBD (a), Pro-MI (b), MI (c), MII (d) 时期的线粒体荧光强度定量; MII时期卵母细胞线粒体均匀分布、核周聚集和无规律聚集的代表性图像 (e) 及其在NG和RPM条件下的比例 (f). 红色表示线粒体
Figure 3. Quantification of fluorescence intensity of mitochondria in GVBD (a), Pro-MI (b), MI (c), MII (d) of oocytes in NG and RPM groups. Representative images of dispersive distribution, perinuclear aggregation and random aggregation of oocytes in MII-stage (e) and proportions under NG and RPM conditions (f). Red represents mitochondria
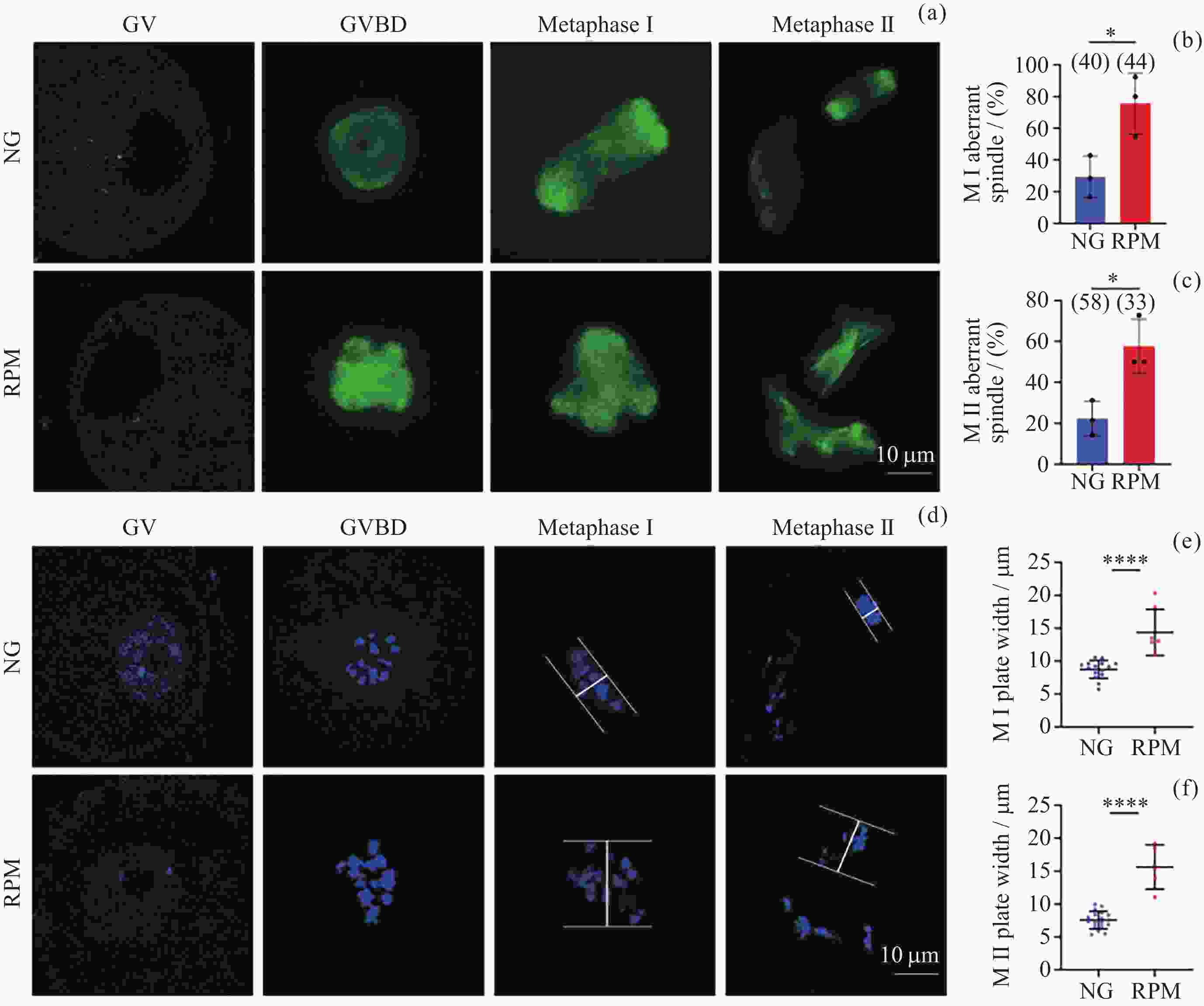
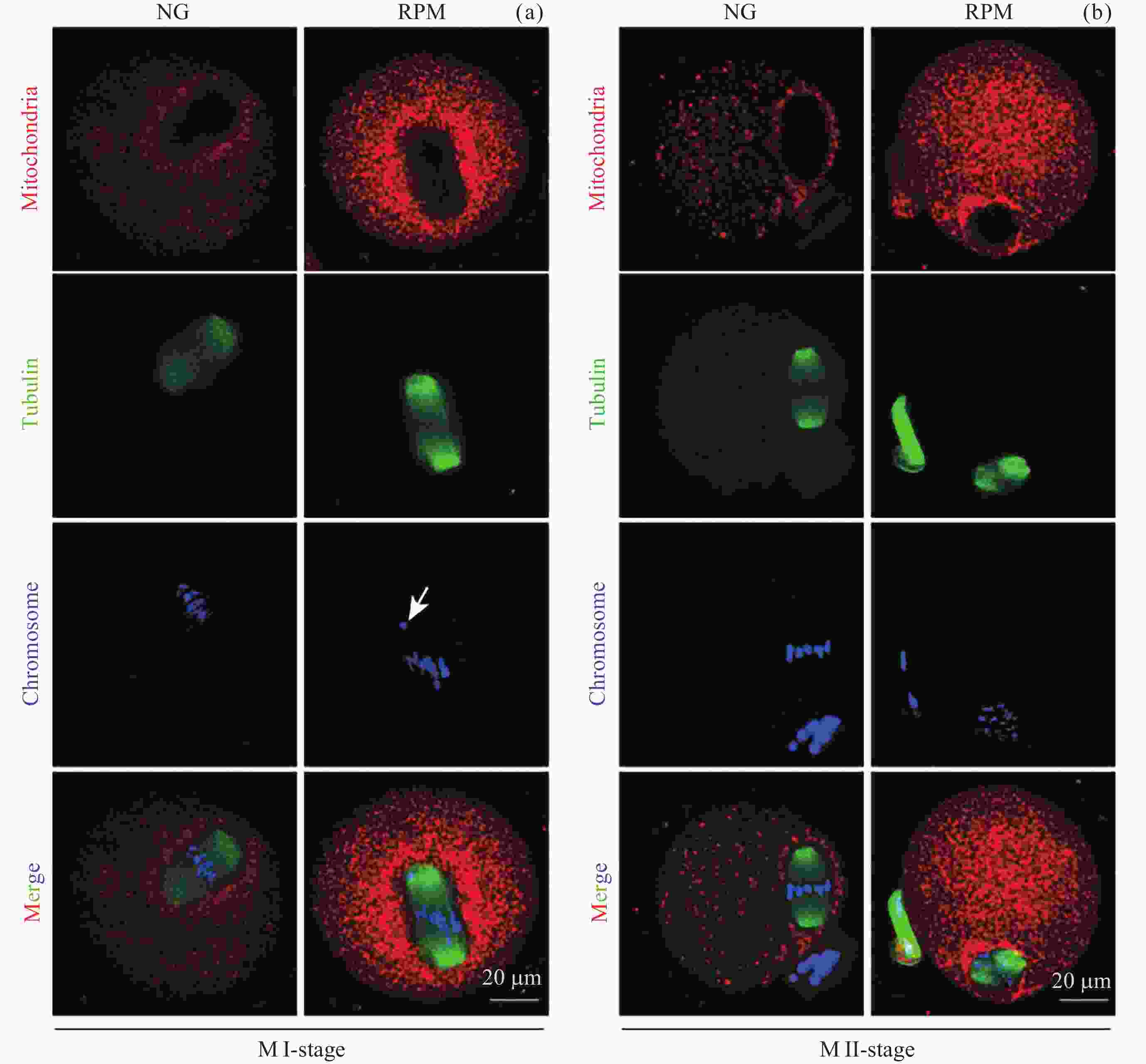
图 4 NG组和RPM组卵母细胞GV, GVBD, Metaphase I, Metaphase II时期纺锤体形态 (a)以及染色体排列 (d) 的代表性图像; NG与RPM条件下MI期卵母细胞纺锤体异常率 (b)和染色体中板宽度 (e) 定量; NG与RPM条件下MII期卵母细胞纺锤体异常率 (c)和染色体中板宽度 (f) 定量. 绿色表示微管蛋白, 蓝色表示染色体
Figure 4. Representative images of spindle morphology (a) and chromosome alignment (d) in GV, GVBD, Metaphase I and Metaphase II stages under NG and RPM conditions, respectively. Quantification of spindle abnormalities (b) and chromosome plate width (e) in MI-stage oocytes under NG and RPM conditions. Quantification of spindle abnormalities (c) and chromosome plate width (f) in MII-stage oocytes under NG and RPM conditions. Green represents tubulin, and blue represents chromosome
-
[1] LÖBRICH M, JEGGO P A. Hazards of human spaceflight[J]. Science, 2019, 364(6436): 127-128 doi: 10.1126/science.aaw7086 [2] RONCA A E, BAKER E S, BAVENDAM T G, et al. Effects of sex and gender on adaptations to space: reproductive health[J]. Journal of Women’s Health, 2014, 23(11): 967-974 doi: 10.1089/jwh.2014.4915 [3] STELLER J G, ALBERTS J R, RONCA A E. Oxidative stress as cause, consequence, or biomarker of altered female reproduction and development in the space environment[J]. International Journal of Molecular Sciences, 2018, 19(12): 3729 doi: 10.3390/ijms19123729 [4] MISHRA B, LUDERER U. Reproductive hazards of space travel in women and men[J]. Nature Reviews Endocrinology, 2019, 15(12): 713-730 doi: 10.1038/s41574-019-0267-6 [5] LEI X H, CAO Y J, MA B H, et al. Development of mouse preimplantation embryos in space[J]. National Science Review, 2020, 7(9): 1437-1446 doi: 10.1093/nsr/nwaa062 [6] LI Yinghui, SUN Yeqing, ZHENG Huiqiong, et al. Recent review and prospect of space life science in China for 40 years[J]. Chinese Journal of Space Science, 2021, 41(1): 46-67 (李莹辉, 孙野青, 郑慧琼, 等. 中国空间生命科学40年回顾与展望[J]. 空间科学学报, 2021, 41(1): 46-67 doi: 10.11728/cjss2021.01.046LI Yinghui, SUN Yeqing, ZHENG Huiqiong, et al. Recent review and prospect of space life science in China for 40 years[J]. Chinese Journal of Space Science, 2021, 41(1): 46-67 doi: 10.11728/cjss2021.01.046 [7] MA Chiyuan, CAO Yujing, DUAN Enkui, et al. The research progress and prospect of mammalian reproduction and embryonic development under space conditions[J]. Scientia Sinica Vitae, 2021, 51(2): 116-125 (马驰原, 曹宇静, 段恩奎, 等. 太空环境下哺乳动物胚胎发育和生殖研究进展及展望[J]. 中国科学: 生命科学, 2021, 51(2): 116-125 doi: 10.1360/SSV-2020-0136MA Chiyuan, CAO Yujing, DUAN Enkui, et al. The research progress and prospect of mammalian reproduction and embryonic development under space conditions[J]. Scientia Sinica Vitae, 2021, 51(2): 116-125 doi: 10.1360/SSV-2020-0136 [8] LI F, YE Y, LEI X H, et al. Effects of microgravity on early embryonic development and embryonic stem cell differentiation: phenotypic characterization and potential mechanisms[J]. Frontiers in Cell and Developmental Biology, 2021, 9: 797167 doi: 10.3389/fcell.2021.797167 [9] BRADBURY P, WU H J, CHOI J U, et al. Modeling the impact of microgravity at the cellular level: implications for human disease[J]. Frontiers in Cell and Developmental Biology, 2020, 8: 96 doi: 10.3389/fcell.2020.00096 [10] BUKEN C, SAHANA J, CORYDON T J, et al. Morphological and molecular changes in juvenile normal human fibroblasts exposed to simulated microgravity[J]. Scientific Reports, 2019, 9(1): 11882 doi: 10.1038/s41598-019-48378-9 [11] THIEL C S, TAUBER S, LAUBER B, et al. Rapid morphological and cytoskeletal response to microgravity in human primary macrophages[J]. International Journal of Molecular Sciences, 2019, 20(10): 2402 doi: 10.3390/ijms20102402 [12] DA SILVEIRA W A, FAZELINIA H, ROSENTHAL S B, et al. Comprehensive multi-omics analysis reveals mitochondrial stress as a central biological hub for spaceflight impact[J]. Cell, 2020, 183(5): 1185-1201. e20 [13] TANAKA K, NISHIMURA N, KAWAI Y. Adaptation to microgravity, deconditioning, and countermeasures[J]. The Journal of Physiological Sciences, 2017, 67(2): 271-281 doi: 10.1007/s12576-016-0514-8 [14] LIN X, ZHANG K W, WEI D X, et al. The impact of spaceflight and simulated microgravity on cell adhesion[J]. International Journal of Molecular Sciences, 2020, 21(9): 3031 doi: 10.3390/ijms21093031 [15] KIM D S, VAQUER S, MAZZOLAI L, et al. The effect of microgravity on the human venous system and blood coagulation: a systematic review[J]. Experimental Physiology, 2021, 106(5): 1149-1158 doi: 10.1113/EP089409 [16] MIGLIETTA S, CRISTIANO L, ESPINOLA M S B, et al. Effects of simulated microgravity in vitro on human metaphase ii oocytes: an electron microscopy-based study[J]. Cells, 2023, 12(10): 1346 doi: 10.3390/cells12101346 [17] KEEFE D, KUMAR M, KALMBACH K. Oocyte competency is the key to embryo potential[J]. Fertility and Sterility, 2015, 103(2): 317-322 doi: 10.1016/j.fertnstert.2014.12.115 [18] DRAGO-FERRANTE R, DI FIORE R, KAROUIA F, et al. Extraterrestrial gynecology: could spaceflight increase the risk of developing cancer in female astronauts? An updated review[J]. International Journal of Molecular Sciences, 2022, 23(13): 7465 doi: 10.3390/ijms23137465 [19] ZHANG S, ZHENG D H, WU Y G, et al. Simulated microgravity using a rotary culture system compromises the in vitro development of mouse preantral follicles[J]. PLoS One, 2016, 11(3): e0151062 doi: 10.1371/journal.pone.0151062 [20] WU C L, GUO X Z, WANG F, et al. Simulated microgravity compromises mouse oocyte maturation by disrupting meiotic spindle organization and inducing cytoplasmic blebbing[J]. PLoS One, 2011, 6(7): e22214 doi: 10.1371/journal.pone.0022214 [21] CHENG K X, FENG X A, YANG C, et al. Simulated microgravity reduces quality of ovarian follicles and oocytes by disrupting communications of follicle cells[J]. NPJ Microgravity, 2023, 9(1): 7 doi: 10.1038/s41526-023-00248-5 [22] YU Y S, DUMOLLARD R, ROSSBACH A, et al. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation[J]. Journal of Cellular Physiology, 2010, 224(3): 672-680 doi: 10.1002/jcp.22171 [23] LEE I W, ADHIKARI D, CARROLL J. Miro1 depletion disrupts spatial distribution of mitochondria and leads to oocyte maturation defects[J]. Frontiers in Cell and Developmental Biology, 2022, 10: 986454 doi: 10.3389/fcell.2022.986454 [24] MICHALETTI A, GIOIA M, TARANTINO U, et al. Effects of microgravity on osteoblast mitochondria: a proteomic and metabolomics profile[J]. Scientific Reports, 2017, 7(1): 15376 doi: 10.1038/s41598-017-15612-1 [25] JIANG M, WANG H M, LIU Z F, et al. Endoplasmic reticulum stress-dependent activation of iNOS/NO-NF-κB signaling and NLRP3 inflammasome contributes to endothelial inflammation and apoptosis associated with microgravity[J]. The FASEB Journal, 2020, 34(8): 10835-10849 doi: 10.1096/fj.202000734R [26] HOPPELER H, FLÜCK M. Plasticity of skeletal muscle mitochondria: structure and function[J]. Medicine and Science in Sports and Exercise, 2003, 35(1): 95-104 doi: 10.1097/00005768-200301000-00016 [27] ADAMS S H, HOPPEL C L, LOK K H, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid β-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women[J]. The Journal of Nutrition, 2009, 139(6): 1073-1081 doi: 10.3945/jn.108.103754 [28] HARASIMOV K, URAJI J, MÖNNICH E U, et al. Actin-driven chromosome clustering facilitates fast and complete chromosome capture in mammalian oocytes[J]. Nature Cell Biology, 2023, 25(3): 439-452 doi: 10.1038/s41556-022-01082-9 -
-





 杨雨馨 女, 2002年1月出生于浙江省嘉兴市, 现就读于中国科学院大学材料科学与光电技术学院/中国科学院深圳先进技术研究院生物医药与技术研究所, 硕士研究生, 研究方向为空间生命科学、空间胚胎发育. E-mail:
杨雨馨 女, 2002年1月出生于浙江省嘉兴市, 现就读于中国科学院大学材料科学与光电技术学院/中国科学院深圳先进技术研究院生物医药与技术研究所, 硕士研究生, 研究方向为空间生命科学、空间胚胎发育. E-mail: 

 下载:
下载: